Kratom derives from Mitragyna speciosa (Korth.), a tropical tree in the genus Mitragyna (Rubiaceae) that also includes the coffee tree. Mitragynine and oxycodone.
Kratom leaf powders, tea-like decoctions, and commercial extracts are taken orally, primarily for health and well-being by millions of people globally. Others take kratom to eliminate opioid use for analgesia and manage opioid withdrawal and use disorder.
There is debate over the possible respiratory depressant overdose risk of the primary active alkaloid, mitragynine, a partial μ-opioid receptor agonist, that does not signal through ß-arrestin, the primary opioid respiratory depressant pathway.
Join the Kratom Lords Family
Sign up to get 10% off on your first order. Stay updated on the latest deals, flash sales, latest Kratom news and more!
Best Sellers
Objectives
Compare the respiratory effects of oral mitragynine to oral oxycodone in rats with the study design previously published by US Food and Drug Administration (FDA) scientists for evaluating the respiratory effects of opioids (Xu et al., Toxicol Rep 7:188–197, 2020).
Methods
Blood gases, observable signs, and mitragynine pharmacokinetics were assessed for 12 h after 20, 40, 80, 240, and 400 mg/kg oral mitragynine isolate and 6.75, 60, and 150 mg/kg oral oxycodone hydrochloride.
Findings
Oxycodone administration produced significant dose-related respiratory depressant effects and pronounced sedation with one death each at 60 and 150 mg/kg. Mitragynine did not yield significant dose-related respiratory depressant or life-threatening effects. Sedative-like effects, milder than produced by oxycodone, were evident at the highest mitragynine dose. Maximum oxycodone and mitragynine plasma concentrations were dose related.
Conclusions
Consistent with mitragynine’s pharmacology that includes partial µ-opioid receptor agonism with little recruitment of the respiratory depressant activating β-arrestin pathway, mitragynine produced no evidence of respiratory depression at doses many times higher than known to be taken by humans.
Introduction
Kratom products are used globally for health and well-being, improved mood, sleep, attention, and coffee-caffeine-like alerting effects (Grundmann, 2017; Henningfield et al. 2018, 2022; Swogger et al. 2022). There is recreational use; however, many people who use opioids recreationally report kratom as a poor substitute for producing opioid-like euphoria but do find it useful to self-manage withdrawal and discontinue opioid use (Coe et al. 2019; Garcia-Romeu et al. 2020; Prozialeck et al. 2019; Swogger et al. 2022). Estimates of incidence and prevalence of intake vary from 2 to 16 million in the United States (US) (American Kratom Association 2019; Covvey et al. 2020; Henningfield et al. 2021; Palamar 2021; Schimmel et al. 2021) and many millions more globally, with the highest prevalence in Southeast Asia and Indonesia where kratom grows in abundance and has been widely consumed for centuries (Brown et al. 2017; Cinosi et al. 2015; Prozialeck et al. 2021; WHO ECDD 2021; Vicknasingam et al. 2010).
People also read: Kratom effects; Can You Overdose On Kratom?
Deaths attributed directly to kratom use in the US and globally are rare and were not documented in Southeast Asia (Prozialeck et al. 2019; Ramanathan and McCurdy 2020). Analyses of kratom-associated deaths found that most of such deaths are more likely attributed to the use of other substances and causes not related to kratom (Gershman et al. 2019; Henningfield et al. 2019; National Institute on Drug Abuse 2020; Olsen et al. 2019).
Provisional estimates from the US Centers for Disease Control and Prevention (CDC) indicated that nearly 76,000 of more than 100,000 drug overdose deaths in 2021 were attributable to opioids (CDC 2021). In October 2021, the US Department of Health and Human Services (US DHHS) announced that drug overdose prevention efforts would include a substantial increase in harm reduction efforts to reduce opioid and other drug overdose deaths (US DHHS 2021). Kratom was not mentioned in the 2021 US DHHS announcement; however, US national surveys indicated that kratom is increasingly taken as a harm reduction strategy by thousands of opioid users to reduce and/or eliminate their opioid use related to pain and/or opioid use disorder (Grundmann 2017; Grundmann et al. 2018; Coe et al. 2019; Garcia-Romeu et al. 2020; Prozialeck et al. 2019, 2020, 2021; Swogger et al. 2022).
Oral mitragynine was investigated because mitragynine is the only alkaloid present at potentially biologically active levels in many widely marketed and consumed kratom products that also include mitragynine isolates. Many products have low or nondetectable concentrations of other alkaloids that may contribute to respiratory and other effects (Chakraborty et al. 2021; Sharma et al. 2019; Sharma and McCurdy 2021). Furthermore, unlike opioids and other substances widely used recreationally that are taken intravenously, insufflated, or inhaled (e.g., O’Brien 2015), kratom is taken almost exclusively by the oral route as dried kratom leaf powder teas, foods, or beverages to mask its bitter unpleasant taste, or leaf powder-filled capsules (Cinosi et al. 2015; Henningfield et al. 2018, 2022; Ramanathan and McCurdy 2020; Singh et al. 2016).
Earlier studies concluded that mitragynine has low respiratory depressant potential as compared to morphine or other µ-opioid agonists but did not include blood oxygen and related blood gas measures. Macko et al. (1972) measured respiratory rate in cats and dogs following morphine and/or codeine or mitragynine administration and found fewer effects from mitragynine. In another recently published study (Hill et al. 2022), 3 to 90 mg/kg mitragynine doses were compared to 3, 10, and 30 mg/kg morphine doses administered by oral gavage to mice tested in plethysmography chambers enabling measurement of minute respiratory volumes. Morphine produced dose-dependent decreases in minute volumes. The maximal effect of 90 mg/kg mitragynine was between that obtained with 10 to 30 mg/kg morphine.
The present study was designed to further evaluate mitragynine’s respiratory effects including effects on blood gases. The same basic approach as FDA utilized in its own studies comparing a broad range of substances to a prototypic respiratory depressing opioid, oxycodone (Xu et al. 2020, 2021) was employed. Oxycodone was selected by the FDA as the prototypic opioid for comparison and the dosing strategy was typical of what FDA often recommends to drug developers, namely inclusion of the therapeutic dose equivalent, and two or more supratherapeutic doses of oxycodone (6.75, 60, and 150 mg/kg).
The rational for selection of the mitragynine doses was to begin with a low dose utilized by other investigators (20 mg/kg), then systematically increase the dose until the highest tolerable dose or the highest chemically feasible dose due to ethical limitations followed by the laboratory on oral gavage (10.0 mL/kg or about 3.5 mL maximum) and limited mitragynine solubility. The laboratory determined that 400 mg/kg was the maximum dose based on solubility in Tween 20 solvent in a volume of approximately 3.5 mL (adjusted individually per animal based on actual body weight). The 400 mg/kg dose is substantially larger than the highest dose employed in most animal studies and is equivalent to 64.5 mg/kg in humans (dividing by 6.2 for allometric scaling). This is many times higher than is consumed by humans, whose typical per serving intake ranges from approximately 0.35 to 2.0 mg/kg per serving (Prozialeck et al. 2020; Singh et al. 2016; Swogger et al. 2022).
Respiratory effect measures were partial pressure carbon dioxide (pCO2), oxygen saturation (sO2), partial pressure oxygen (pO2), bicarbonate (HCO3), pH, and lactate in rat blood after oral mitragynine and oxycodone. Observable signs of drug effects for 12 h after dosing by veterinary-trained technicians with extensive experience in rat safety and pharmacokinetics studies are also reported (see details in the “Methods” section). The full pharmacokinetics of oxycodone and mitragynine will be reported elsewhere, whereas the present article reports maximum concentrations (Cmax) and time of Cmax (Tmax), confirming dose-dependent increases in oxycodone and mitragynine.
Methods
Animals
Adult male Sprague Dawley rats (n = 48) approximately 80 days old weighed between 350 and 375 g at the time of surgical placement of carotid artery catheters at Charles River Laboratories. Actual body weights on dosing days ranged from 323.0 to 372.8 g. Animals were acclimated for 1 day at the CARE research facility due to concerns about the patency of the arterial catheters for collecting the primary blood gas and pharmacokinetic parameters. To ensure catheter patency, the catheters were flushed with heparinized saline every other day. A small amount of heparinized saline was left in the catheter as a “locking solution” to prevent clots from forming.
Rats were single-housed in a temperature-controlled room (20–26 °C) under a 12:12 h light/dark cycle, with 15–70% humidity and corn cob bedding. Animals had ad libitum access to Teklad Rodent Chow 2018 and filtered tap water, and no other medications were administered.
Animal care procedures were approved by the CARE Research Institutional Animal Care and Use Committee and animal welfare complied with the US Department of Agriculture’s (USDA) Animal Welfare Act (9 CFR Parts 1, 2, and 3), the Guide for the Care and Use of Laboratory Animals (National Academy of Sciences 2011), and laboratory standard operating procedures. Animals were euthanized and disposed of without necropsy in accordance with accepted American Veterinary Medical Association guidelines (Underwood and Anthony 2020).
Drug preparation and administration
All drug doses were administered by oral gavage in maximum volumes of 10 mL/kg based on pre-session body weights for each animal following the ethical limitations of the laboratory. Table 1 includes doses and the number of days from delivery of the animals to Care Research from Charles River Labs that each dose was administered. Oxycodone hydrochloride from Sigma-Aldrich (Product Number O1378-500 mg) was dissolved in sterile water the morning of each test day. Doses were based on the weight of 100% pure oxycodone hydrochloride (6.75, 60.0, and 150.0 mg/kg). Mitragynine, 99.9% pure, extracted from Mitragyna Speciosa from Coryn Pharmaceuticals (Product Number 5057), was dissolved, or suspended in 20% Tween 20, because it is not water soluble. Mitragynine dissolved poorly in 20% Tween 20 above concentrations of roughly 10 to 15 mg/mL, requiring mitragynine doses to be prepared individually for each animal immediately before testing using vortex and sonication. To achieve the 240 and 400 mg/kg doses, the solution was in the form of a suspension, mixed well by vortex, and administered within 1 min of each preparation. Mitragynine was stored at room temperature in the dark and used within 62 days of receipt, with study completion within seven days from the first to the last dose.
Table 1 Administered doses, sequence of drug dosing, and the day following rat receipt the session was conducted. Test sessions were initiated between 7:30 and 8:30 AM during the light cycle.
| Group # drug dose mg/kg* | Sequence | Days after rats delivered for session |
|---|---|---|
| 1. Oxycodone 6.75 | 5 | 8** |
| 2. Oxycodone 60 | 4 | 4 |
| 3. Oxycodone 150 | 3 | 2 |
| 4. Mitragynine 20 | 1 | 1 |
| 5. Mitragynine 40 | 1 | 1 |
| 6. Mitragynine 80 | 2 | 2 |
| 7. Mitragynine 240 | 5 | 8** |
| 8. Mitragynine 400 | 4 | 4 |
*Doses were calculated for each animal based on its pre-session body weight
**Blood gas analyzer failure required testing after a 4-day washout
The three initial mitragynine doses (20, 40, and 80 mg/kg) were the same as in earlier rat studies (Avery et al. 2019; de Moraes et al. 2009; Jagabalan et al. 2019), where data suggested maximum tolerable doses might be several times higher than 80 mg/kg. Drs. Henningfield and Huestis were briefed by the study director (Dr. Magnuson) 12 h after dosing each test day to determine if the next provisionally planned dose was likely tolerable and/or if it should be adjusted to document the dose–response curve as fully as possible. Because there were no observed behavioral effects, adverse events, or changes in blood gases at 80 mg/kg, the next dose was adjusted to 240 mg/kg, and the highest dose set to the maximum chemically feasible dose of 400 mg/kg.
Test session sequence
Two groups were tested each day. Table 1 shows the dosing sequence and the days after the animals were delivered to the laboratory. Mitragynine doses increased from the lowest to highest doses, while oxycodone doses descended from the highest to lowest doses. Due to a malfunction of the blood gas analyzer, animals dosed with oxycodone 60 mg/kg and mitragynine 240 mg/kg had blood gases measured for only 2 h. No data from these doses were utilized, and the animals underwent a 4-day washout period. The oxycodone 60 mg/kg dose was administered the next day to naïve rats. Animals originally dosed with 60 mg/kg oxycodone were re-dosed with 6.75 mg/kg oxycodone after the 4-day washout, and animals originally dosed with 240 mg/kg mitragynine were re-dosed with 240 mg/kg mitragynine after the 96 h washout. Protocol changes were approved by the ethical committee and permitted rats adequate recovery from the few blood collections performed.
Test session protocol
Animals were removed from their home cages for weighing at approximately 6 am each day and at 24 h post-dose, for drug administration and blood collections, and immediately returned to their home cages. Blood collections occurred pre-dose between 7:30 and 8:30 am and 1, 2, 4, 6, 8, and 12 h post-dose. A total of 300 µL whole blood (EDTA anticoagulant) was drawn at each time point (2.1 mL total). Approximately 100 µL was for blood gas analysis with a portable blood gas testing device (VetScan i-STAT Alinity V Handheld Analyzer with CG4 + cartridges (Zoetis/Abaxis/Abbott)) at 7 time points following procedures described by Xu et al. (2020). Blood gases were measured within 10 min after each blood collection.
A single blood gas measurement obtained data for all blood gas parameters at each time point for each animal and at each dose. A two-way ANOVA with multiple comparisons was performed, with the Dunnett test accounting for multiple comparisons, and significance set to p <
0.05. The remaining 200 µL blood was processed immediately for subsequent plasma oxycodone or mitragynine analyses, with storage at − 60 °C or below. Arterial ports were flushed with saline-heparin after each blood draw and every other day.
Observable signs were collected by the two veterinary-trained session monitors who identified and recorded adverse events and deviations from normal behavior. Observable signs included general activity (lethargy, impaired motor function, and righting response), response to stimulus (finger snap or clap near the animal), abnormal behaviors such as bracing (defined as a rigid posture with limbs slightly splayed), and apparent increases or decreases in respiration from normal/control. These items were given special attention as effects commonly observed in mu-agonist opioid studies, and our interest in comparing mitragynine’s effects to those of oxycodone, a prototypical mu-agonist opioid. Observations were conducted at each blood collection and monitored closely following dosing, and anytime an abnormality was observed by one of the two veterinary-trained session monitors. Observations were detailed in writing by the technician who made them. The technician observers were not blinded to treatments.
Six animals received each dose; 200 μL (K2EDTA) blood was processed to obtain plasma (~ 100 μL) for pharmacokinetic analysis. A total of 336 samples were analyzed for oxycodone and mitragynine in two separate validated LC–MS/MS assays. Pharmacokinetic analyses were performed with a non-compartment model (WinNonlin Phoenix professional, Version 8.3, Certara, Princeton, NJ) to determine pharmacokinetic parameters. The maximum plasma concentration (Cmax) and time of maximum plasma concentration (Tmax) following 6.75, 60, and 150 mg/kg oxycodone and 20, 40, 80, 240, and 400 mg/kg/d mitragynine group are presented. Due to space limitations and the many pharmacodynamic findings in this manuscript, a separate report will describe complete oxycodone and mitragynine pharmacokinetics.
Results
Oxycodone and mitragynine pharmacokinetics
Table 2 shows the maximum observed plasma concentrations (Cmax) for oxycodone and mitragynine. Cmax increased by dose from 20 to 400 mg/kg, but each dose increase did not produce a significant increase based on a one-way ANOVA with Tukey’s post hoc analysis and a significance value of p < 0.05. Mitragynine Cmax results were significantly different between the 20 and 40 mg/kg doses and the 240 and 400 mg/kg mitragynine doses, and the 80 mg/kg dose and the 400 mg/kg dose. Although the 240 mg/kg mean Cmax was 5601 and the 400 mg/kg mean Cmax was 7982 ng/mL, this increase was not statistically significant.
Table 2 Median maximum plasma concentrations (Cmax), with the minimum and maximum plasma concentration observed by drug and dose
| Dose mg/kg | n | Median Cmax ng/mL | Range ng/mL |
|---|---|---|---|
| Oxycodone | |||
| 6.75 | 6 | 33.5 | 10.7–51.5 |
| 60 | 6 | 39.9 | 34.5–1179 |
| 150 | 5* | 131 | 93.2–5532 |
| Mitragynine | |||
| 20 | 6 | 723 | 317–1010 |
| 40 | 6 | 1720 | 1043–2032 |
| 80 | 6 | 2497 | 1538–3125 |
| 240 | 4** | 5182 | 3783–7444 |
| 400 | 6 | 7513 | 4404–10,096 |
*One animal’s data were not included in the analysis due to incomplete washout prior to redosing
**Another animal’s data were not included in the analysis due to incomplete washout prior to redosing and one eliminated due to extreme outlier for Cmax
The maximum plasma concentration generally occurred within 1–2 h (Tmax) but varied across animals, occurring as late as 12 h in some animals. Three animals were not included in the pharmacokinetic or pharmacodynamic analyses due to two animals not achieving concentrations below the limit of quantification (one in 150 mg/kg oxycodone group and one in the 240 mg/kg mitragynine group) after a 4-day washout period prior to redosing. One animal in the 240 mg/kg mitragynine group had a catheter blockage and tail blood was collected at time points 6, 8, and 12 h; however, for unexplained reasons, the blood analytic result was an extreme outlier for Cmax at 23,425 ng/mL—a concentration threefold higher than any obtained with the 400 mg/kg dose. This result failed the outlier test and was not included in any analyses.
Respiratory blood gas results
Figure 1 presents the change from pre-dose baseline levels over time of oxycodone and mitragynine on the partial pressure of carbon dioxide (pCO2), which Xu et al. (2021) suggested is more sensitive than partial pressure of oxygen (pO2). None of the three blood gas parameters significantly deviated from baseline at the therapeutic 6.75 mg/kg oxycodone dose. The pCO2 was significantly increased relative to baseline at 1, 4, and 6 h for the 60 mg/kg oxycodone dose and at 1, 2, and 4 h for the 150 mg/kg oxycodone dose. There was a rapid decrease in sO2 and pO2 following the 60 and 150 mg/kg oxycodone doses, with the sO2 significantly depressed at 1, 4, and 6 h for the 60 mg/kg oxycodone dose and 1, 2, 4, and 6 h for the 150 mg/kg oxycodone dose, while no statistically significant decreases in pO2 were observed for either 60 or 150 mg/kg oxycodone. By 8 h post-drug administration, pCO2, sO2, and pO2 were no longer significantly different from baseline for higher oxycodone doses. There were no significant differences in pCO2, sO2, and pO2 following 20, 40, 80, 240, or 400 mg/kg mitragynine from baseline to 12 h.
Figure1
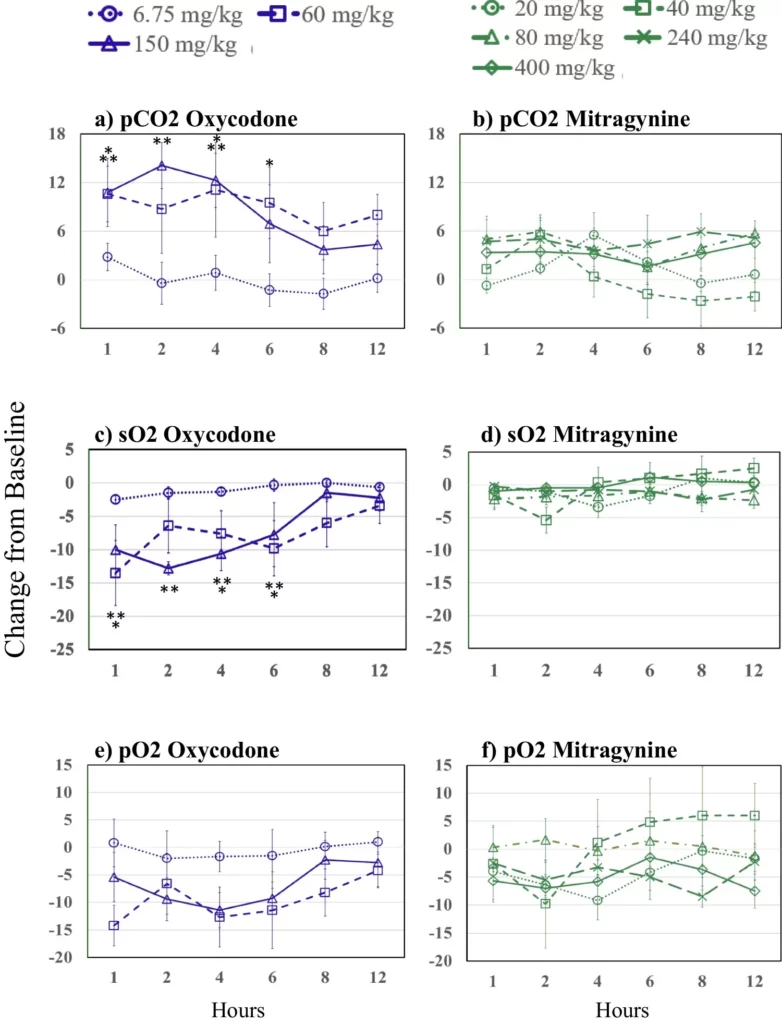
Changes from baseline over time following oxycodone in a partial pressure of carbon dioxide (pCO2), c oxygen saturation (sO2), and e partial pressure of oxygen (pO2); and changes from baseline over time following mitragynine b partial pressure of carbon dioxide (pCO2), d oxygen saturation (sO2), and f partial pressure of oxygen (pO2). Asterisks indicate significant differences from baseline (p≤0.05, one asterisks (*) 60 and two asterisks (**) 150 mg/kg oxycodone). Oxycodone is shown in purple. 6.75 mg/kg, dotted lines and circles; 60 mg/kg, dashed lines and squares; 150 mg/kg, solid lines and triangles. Mitragynine is shown in green; 20 mg/kg, dotted lines and circles; 40 mg/kg, short dashes and squares; 80 mg/kg, dashed and dotted lines and triangles; 240 mg/kg, long dashed lines and Xs; 400 mg/kg, solid lines and diamonds. Figure1 Source
Figure 2 presents oxycodone and mitragynine effects on blood lactate, bicarbonate (HCO3), and pH. No mitragynine or oxycodone dose produced any statistically significant changes in HCO3 concentrations for 12 h after drug administration with the exception of the 400 mg/kg at 12 h post-drug administration. There were no significant changes from baseline in blood lactate following any oxycodone or mitragynine dose. Following 150 mg/kg oxycodone, the pH dropped significantly compared to baseline from 1–4 h and at 1 h after the 60 mg/kg oxycodone dose. There were no significant changes from baseline in pH after any mitragynine dose.
Figure2
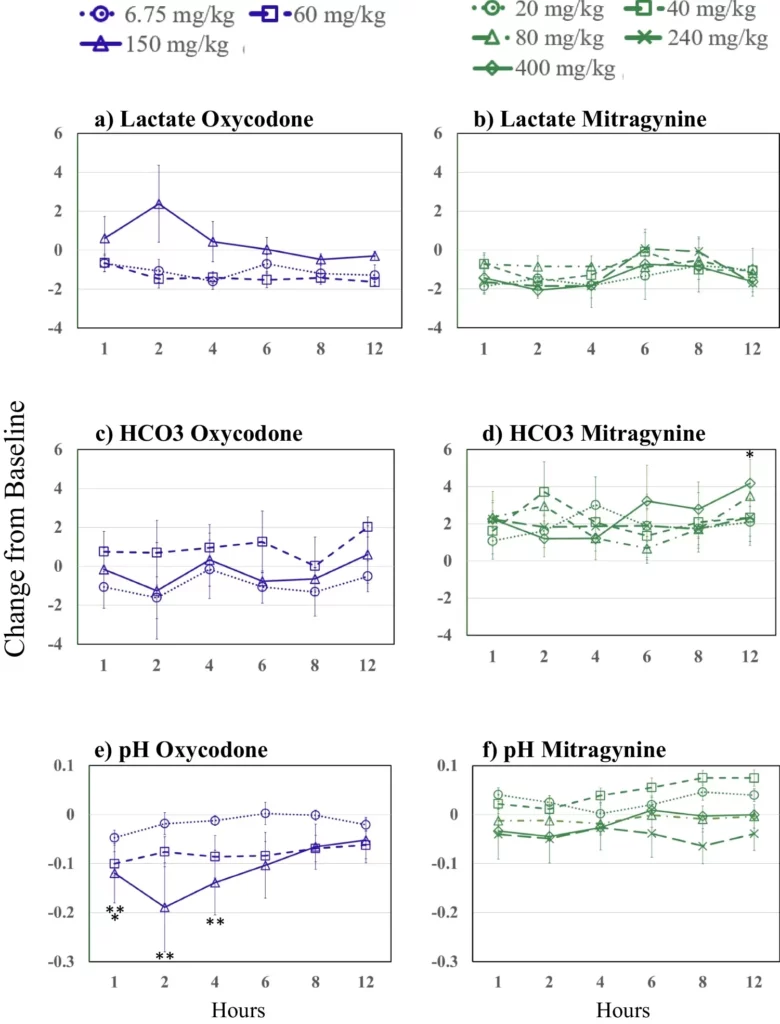
Changes from baseline over time and by dose (see key) following oxycodone on the left side panels (blue) and mitragynine on the right side panels (green) for lactate (top panels), bicarbonate (HCO3) (middle panels), and pH (bottom panels). Asterisks indicate significant differences from baseline (p≤0.05, one asterisk (*) 60 and two asterisks (**) 150 mg/kg oxycodone). Figure2 Source
Observable signs including potential drug-related adverse effects
There was no evidence of opioid or other drug effects or any behavioral observation in any of the animals receiving 6.75 mg/kg oxycodone or 20, 40, 80, and 240 mg/kg mitragynine that was visibly apparent to staff monitoring the animals.
Following 60 and 150 mg/kg oxycodone, the trained observers recorded numerous reports of behavioral effects including decreased activity, lethargy, apparently decreased respiration, and impairments to motor function. Four of the six animals at 60 mg/kg oxycodone showed overt signs of opioid exposure including pronounced behavioral effects and lethargy typical of opioids. One animal receiving 60 mg/kg oxycodone died between the 2 and 4 h time points, apparently as a result of the animal pulling its catheter and causing internal bleeding. These data were not used in the results. Four of the five remaining animals in the 60 mg/kg group appeared normal at 24 h, while one animal was found dead at 7:53 AM the next morning, almost 24 h following drug exposure.
The 150 mg/kg oxycodone dose produced notably stronger behavioral effects and lethargy typical of opioids with observable signs occurring in all 6 animals at 1–2 h. At 4 h post-dose, 3 of the 6 animals appeared normal. However, one of those animals was subsequently found dead approximately 5 h post-drug exposure. Two of the remaining 5 animals continued to exhibit behavioral signs at 6, 8, and 12 h, while all others returned to normal by 6 h. All 5 remaining animals appeared normal at 24 h.
The 400 mg/kg mitragynine dose produced milder observable signs than 60 and 150 mg/kg oxycodone. These observations included alteration of motor behavior, lethargy, and/or incoordination in 5 of 6 animals, with no observed abnormal behavior in the sixth animal. The slowed behavior suggested potentially decreased respiratory rate at 2 and 4 h in five animals.
Approximately 40 min after the 400 mg/kg mitragynine dose, one animal displayed “full body spasms” when picked up to be removed from the home cage for blood collection. Under observation (away from home cage) at approximately 1 h post-mitragynine dose, the animal exhibited what was described as a seizure-like activity for approximately 30 s that was described as moderate. No further seizure-like behavior was observed.
Discussion
The purpose of this study was to evaluate the respiratory effects of kratom’s primary alkaloid, mitragynine, in comparison to oxycodone, a prototypic opioid that reliably produces dose-related respiratory depression and death in humans, rats, and other species. As far as we are aware, this is the first study of mitragynine’s respiratory effects that followed the approach published previously by Xu et al. (2020, 2021) that utilized blood gas assessment of respiratory function. The respiratory effects of a 20-fold range of mitragynine doses were compared to those of therapeutic and supratherapeutic doses of oxycodone for comparative assessments of the respiratory effects of drugs. The main finding was that mitragynine produced no evidence of respiratory depression at doses many times higher than typical human doses, whereas oxycodone produced the expected dose-related respiratory depressant effects consistent with its strong morphine-opioid (i.e., µ-opioid) receptor-mediated effects. These findings are consistent with mitragynine’s pharmacology that includes partial µ-opioid receptor agonism with little recruitment of the respiratory depressant activating β-arrestin pathway (Kruegel et al. 2016, 2019; Váradi et al. 2016).
Observable signs and blood pharmacokinetics confirmed that the administered doses produced the dose-related pharmacodynamic and pharmacokinetic results expected, namely, higher plasma concentrations and stronger behavioral effects.
The blood gas assessments revealed striking dose and drug-related effects. We found that oxycodone, in the same oral doses used by Xu et al. (2020), did not produce significant respiratory depressant effects at the therapeutic equivalent dose of 6.75 mg/kg, and had strongly depressant effects at 60 mg/kg and more severe and longer lasting effects at 150 mg/kg. In contrast, mitragynine did not produce dose-related respiratory depression or life-threatening effects at any dose, and there were no dose-related trends in blood oxygen or carbon dioxide. Consistent with the conclusion of Xu et al. (2021), pCO2 appeared to be a more sensitive measure of the respiratory depressant effects of oxycodone than blood oxygen measures; however, none of the blood gas measures showed dose-related changes associated with mitragynine administration.
This is not the first study to conclude that mitragynine does not produce opioid-like dose-dependent respiratory depressant effects. This was also demonstrated by Macko et al. (1972), and Hill et al. (2022) found no respiratory depressant effect at 3 mg/kg but did find some respiratory depression at 10 mg/kg, which they reported did not change at higher doses. Whether this difference from the present finding is unique to mice, measurement techniques or another factor is not known. In addition, Váradi et al. (2016, p. 7) reported that, in contrast to the dose-dependent respiratory depressant effects of morphine, a mitragynine analog “shows a lower propensity to cause respiratory depression and constipation compared with the canonical opioid, morphine.”
The human equivalent doses (HEDs) of those tested in this rat study are based on estimated body surface area dividing the rat doses by 6.2 (Nair and Jacob 2016). Based on this conversion, the HEDs of 20, 40, 80, 240, and 400 mg/kg are 3.2, 6.6, 12.9, 38.7, and 64.5 mg/kg respectively. Manufacturers of mitragynine-containing kratom extracts state that about 25 mg per serving or about 0.35 mg/kg in a 70 kg human is the minimal amount that provides sufficient self-reported benefits by consumers to support repeat sales. Some marketed products are labeled with this amount and commonly marketed products in Southeast Asia in the form of tea-like decoctions typically contain 22.5 to 75 mg but with wide variation across marketed products (Brown et al. 2017; Cinosi et al. 2015; Prozialeck et al. 2020; WHO ECDD 2021). Thus, it appears that the mitragynine doses administered in the present study range from the high end of consumer use (e.g., at the 20 mg/kg dose) to levels many times higher than would be voluntarily consumed or considered desirable or tolerable, regardless of their apparent limited effects on respiratory function. Some people who are self-treating chronic pain and/or using to maintain opioid abstinence may consume larger doses of 2–5 mg/kg 2–4 times per day (Cinosi et al. 2015; Figura et al. 2020; European Monitoring Centre 2015; McCurdy et al. 2020; Ramanathan and McCurdy 2020; Sharma and McCurdy 2021; Swogger et al. 2022; WHO ECDD 2021).
Self-report data posted on internet websites were summarized in several kratom use reviews (e.g., Cinosi et al 2015; Henningfield et al. 2018, 2022; Swogger and Walsh 2018; Veltri and Grundmann 2019). These data suggest that kratom intake is typically limited by non-life-threatening but discomfort-producing gastrointestinal symptoms and/or undesirable lethargy. Furthermore, for most kratom consumers, higher doses do not produce the powerful euphoria-like highs sought by people who recreationally use opioids, cocaine, and amphetamine and that is an incentive for the frequent dose escalation that contributes to the high risk of overdose deaths associated with such drugs (Swogger et al. 2022; Swogger and Walsh 2018; Henningfield et al. 2018, 2022).
An important caveat to the foregoing observations is that it should not be concluded that kratom is without life-threatening risks under some conditions and in some people, any more than such assumption should be made for any dietary or consumer product. It is possible, if not plausible, that in some yet-to-be documented combination with other drugs or underlying health conditions, high-dose kratom consumption could pose a serious risk. Therefore, it is prudent, as discussed elsewhere (e.g., Swogger et al. 2022) to not consume kratom with other drugs or in high dosages. For example, buprenorphine is a partial agonist that is approved for the treatment of pain and opioid use disorder and also produces little evidence of life-threatening depression over a broad range of doses. Buprenorphine did not produce life-threatening respiratory depression in humans even after a 16 mg intravenous dose (Umbricht et al. 2004; Huestis et al. 2013). However, buprenorphine is associated with overdose deaths when combined with benzodiazepines and other sedative-hypnotic drugs (Pergolizzi et al. 2015; Pergolizzi and Raffa 2019; Schuman-Olivier et al. 2013).
The absence of evidence for respiratory depressant effects in rats documented here does not rule out the possibility of respiratory depressant effects in some human conditions and in combination with other substances. Mitragynine in isolation and kratom produce diverse opioid and nonopioid mediated effects that warrant further study (Ahmad et al. 2022; Harun et al. 2022; Behnood-Rod et al. 2020; Hemby et al. 2019; Henningfield et al. 2018, 2022; Reeve et al. 2020; Sharma and McCurdy 2021; Wilson et al. 2020; Yue et al. 2018). However, as compared to opioids and other substances that contributed to the 2021 annualized rate of 108,000 drug overdose deaths, the relative risk of kratom appears to be far lower than that of opioids, methamphetamine and cocaine, alcohol, and many other substances of abuse (CDC 2021; Giroir 2018; Henningfield et al. 2019; United Nations Commission on Narcotic Drugs 2021).
The risk of kratom producing seizures is real but low and extensive surveillance to determine the conditions under which kratom may increase the risk may be necessary. For example, Boyer et al. (2008) reported a seizure in a person with no history of seizures following the ingestion of 100 mg modafinil combined with an unknown amount of kratom. Follow-up evaluation including computerized tomography and magnetic resonance brain imaging was normal. Modafinil itself can lower the seizure threshold and possibly cause seizures (Artsy et al. 2012; Bahramnjead et al. 2018). Thus, it is not known if kratom contributed but an interaction cannot be ruled out.
Current findings in the context of recent advances in understanding the pharmacology of mitragynine
The finding that a broad range of mitragynine doses did not produce respiratory depression is not novel in itself because there are earlier studies of mitragynine across diverse doses and conditions, albeit at generally lower doses of 20–56 mg/kg in rats. These studies did not as intensively evaluate respiratory effects, and most did not include a pharmacokinetic assessment of mitragynine in comparison to a prototypic opioid. Several studies administered mitragynine to mice and dogs in experimental models with behaviorally functioning animals in conditioned place preference, self-administration, and intracranial self-stimulation models, reviewed recently by Henningfield et al. (2022), Ramanathan and McCurdy (2020), Sharma and McCurdy (2021), and WHO ECDD (2021). None of these studies reported life-threatening effects of mitragynine of 20–56 mg/kg and higher (e.g., Avery et al. 2019; Behnood-Rod et al. 2020; Maxwell et al. 2020; Obeng et al. 2021; Gutridge et al. 2020; Hassan et al. 2021; Kamble et al. 2021; Suhaimi et al. 2021; Todd et al. 2020). For example, a pharmacokinetics and safety study in beagle dogs reported a “mild transient sedation” lasting about 2–4 h after 5 mg/kg oral mitragynine but without clinically significant effects on vital signs (Maxwell et al. 2020). Váradi et al. (2016) found that morphine, but not a mitragynine analog, dose-dependently decreased respiratory rate in rats.
The main strengths of the present study include its dose-related comparison of oxycodone to substantially higher doses of mitragynine than were tested in most other studies and by close adherence to the protocol used in two published studies for evaluating respiratory effects (Xu et al. 2020, 2021). Its main limitation with respect to its goals was that the limit of tolerability, as defined by life-threatening effects, was not discovered and was limited by dosing restrictions. Future studies will consider alternative oral dosing approaches to enable the administration of higher doses. It is also possible that the two groups of animals that were not mitragynine naïve due to the blood gas analyzer failure were affected by their prior mitragynine exposure despite the 4-day washout period. Nor does this single ascending dose (SAD) approach address the effects of chronic dosing as can be informed by multiple ascending dose (MAD) studies. Future studies will help better understand the generalizability of the present findings, although the overall findings that the respiratory effects of mitragynine are weak albeit at lower doses as compared to morphine-like opioids are not novel. It also is important to evaluate the effects of mitragynine in combinations with drugs commonly taken with kratom including opioids, sedatives, alcohol, and stimulants. Further studies of mitragynine and other kratom constituents and analytes are clearly warranted to continue to advance science and public health.
Sources
Henningfield, J.E., Rodricks, J.V., Magnuson, A.M. et al. Respiratory effects of oral mitragynine and oxycodone in a rodent model. Psychopharmacology (2022). https://doi.org/10.1007/s00213-022-06244-z
Autor References
- Ahmad I, Prabowo WC, Arifuddin M, Fadraersada J, Indriyanti N, Herman H, Purwoko RY, Nainu F, Rahmadi A, Paramita S, Kuncoro H, Mita N, Narsa AC, Prasetya F, Ibrahim A, Rijai L, Alam G, Mun’im A, Dej-adisai S, (2022) Mitragyna species as pharmacological agents: from abuse to promising pharmaceutical products. Life 12:193. https://doi.org/10.3390/life12020193
- American Kratom Association (2019) The increase in consumer use of kratom in the United States, June 2019. https://assets.website-files.com/61858fcec654303987617512/619dd9d68bd48315873c4952_Kratom_Population_2019.pdf. Accessed 19 Oct 2022
- Artsy E, McCarthy DC, Hurwitz S, Pavlova MK, Dworetzky BA, Lee JW (2012) Use of modafinil in patients with epilepsy. Epilepsy Behav 23(4):405–408. https://doi.org/10.1016/j.yebeh.2012.02.011
- Avery BA, Boddu SP, Sharma A, Furr EB, Leon F, Cutler SJ (2019) McCurdy CR (2019) Comparative pharmacokinetics of mitragynine after oral administration of Mitragyna speciosa (Kratom) leaf extracts in rats. Planta Med 85(4):340–346. https://doi.org/10.1055/a-0770-3683
- Bahramnjead E, Kazemi Roodsari S, Rahimi N, Etemadi P, Aghaei I, Dehpour AR (2018) Effects of modafinil on clonic seizure threshold induced by pentylenetetrazole in mice: involvement of glutamate, nitric oxide, GABA, and serotonin pathways. Neurochem Res 43:2025–2037. https://doi.org/10.1007/s11064-018-2623-7
- Behnood-Rod A, Chellian R, Wilson R, Hiranita T, Sharma A, Leon F, McCurdy CR, McMahon LR, Bruijnzeel AW (2020) Evaluation of the rewarding effects of mitragynine and 7-hydroxymitragynine in an intracranial self-stimulation procedure in male and female rats. Drug Alcohol Depend 215:108235. https://doi.org/10.1016/j.drugalcdep.2020.108235
- Boyer EW, Babu KM, Adkins JE, McCurdy CR, Halpern JH (2008) Self-treatment of opioid withdrawal using kratom (Mitragynia speciosa korth). Addiction 103(6):1048–1050. https://doi.org/10.1111/j.1360-0443.2008.02209.x
- Brown PN, Lund JA, Murch SJ (2017) A botanical, phytochemical and ethnomedicinal review of the genus Mitragyna korth: implications for products sold as kratom. J Ethnopharmacol 202:302–325. https://doi.org/10.1016/j.jep.2017.03.020
- Centers for Disease Control and Prevention (CDC), National Center for Health statistics (2021) Drug Overdose Deaths in the U.S. Top 100,000 Annually. https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2021/20211117.htm. Accessed 8 March 2022
- Chakraborty S, DiBerto JF, Faouzi A, Bernhard SM, Guttridge AM Ramsey, S, et al (2021) A novel mitragynine analog with low efficacy mu opioid receptor agonism displays antinociception with attenuated adverse effects. J Med Chem 64(18):13873–13892
https://doi.org/10.1021%2Facs.jmedchem.1c01273 - Cinosi E, Martinotti G, Simonato P, Singh D, Demetrovics Z, Roman-Urrestarazu A, Bersani FS, Vicknasingam B, Piazzon G, Li JH, Yu WJ, Kapitány-Fövény M, Farkas J, Di Giannantonio M, Corazza O (2015) Following “the roots” of Kratom (Mitragyna speciosa): the evolution of an enhancer from a traditional use to increase work and productivity in Southeast Asia to a recreational psychoactive drug in Western countries. Biomed Res Int 2015:968786. https://doi.org/10.1155/2015/968786
- Coe MA, Pillitteri JL, Sembower MA, Gerlach KK, Henningfield JE (2019) Kratom as a substitute for opioids: results from an online survey. Drug Alcohol Depend 202:24–32. https://doi.org/10.1016/j.drugalcdep.2019.05.005
- Covvey JR, Vogel SM, Peckham AM, Evoy KE (2020) Prevalence and characteristics of selfreported kratom use in a representative US general population sample. J Addict Dis 38(4):506–513
https://doi.org/10.1080%2F10550887.2020.1788914 - de Moraes NV, Moretti RA, Furr EB III, McCurdy CR, Lanchote VL (2009) Determination of mitragynine in rat plasma by LC–MS/MS: application to pharmacokinetics. J Chromatogr B Analyt Technol Biomed Life Sci 877(24):2593–2597. https://doi.org/10.1016/j.jchromb.2009.06.023
- Epilepsy Foundation (2020) Triggers of seizures: over-the-counter medications. https://www.epilepsy.com/learn/triggers-seizures/over-counter-medications. Accessed 8 March 2022
- European Monitoring Centre for Drugs and Drug Addiction (2015) Kratom drug profile. https://www.emcdda.europa.eu/publications/drug-profiles/kratom_en. Accessed 7 March 2022
- Figura C, Barrett M, Atkinson TJ (2020) A kratom primer: miracle medicine or herb of abuse? Pract Pain Manag 20(5). https://www.practicalpainmanagement.com/treatments/pharmacological/non-opioids/kratom-primer-miracle-medicine-herb-abuse. Accessed 8 March 2022
- Garcia-Romeu A, Cox DJ, Smith KE, Dunn KE, Griffiths RR (2020) Kratom (Mitragyna speciosa): user demographics, use patterns, and implications for the opioid epidemic. Drug Alcohol Depend 208:107849. https://doi.org/10.1016/j.drugalcdep.2020.107849
- Gershman K, Timm K, Frank M, Lampi L, Melamed J, Gerona R, Monte AA (2019) Deaths in Colorado attributed to kratom. N Engl J Med 380(1):97–98. https://doi.org/10.1056/NEJMc1811055
- Giroir BP (2018) Letter from the Assistant Secretary of Health to the Administrator of the Drug Enforcement Administration to Rescind Previous Support to Permanently Place Mitragynine and 7-hydroxymitragynine in Schedule I of the Controlled Substances Act 2018. https://static1.squarespace.com/static/54d50ceee4b05797b34869cf/t/60145eab6df59e7e36a7cfc1/1611947693695/dhillon-8.16.2018-response-letter-from-ash-radm-giroir.pdf. Accessed 8 March 2022
- Grundmann O (2017) Patterns of Kratom use and health impact in the US—results from an online survey. Drug Alcohol Depend 176:63–70. https://doi.org/10.1016/j.drugalcdep.2017.03.007
- Grundmann O, Brown PN, Henningfield J, Swogger M, Walsh Z (2018) The therapeutic potential of kratom. Addiction 113(10):1951–1953. https://doi.org/10.1111/add.14371
- Gutridge AM, Robins MT, Cassell RJ, Uprety R, Mores KL, Ko MJ, Pasternak GW, Majumdar S, van Rijn RM (2020) G protein-biased kratom-alkaloids and synthetic carfentanil-amide opioids as potential treatments for alcohol use disorder. Br J Pharmacol 177(7):1497–1513. https://doi.org/10.1111/bph.14913
- Harun N, Kamaruzaman NA, Mohamed Sofian Z, Hassan Z (2022) Mini review: potential therapeutic values of mitragynine as an opioid substitution therapy. Neurosci Lett 773:136500. https://doi.org/10.1016/j.neulet.2022.136500
- Hassan R, Sreenivasan S, Müller CP, Hassan Z (2021) Methadone, buprenorphine, and clonidine attenuate mitragynine withdrawal in rats. Front Pharmacol 12:708019. https://doi.org/10.3389/fphar.2021.708019
- Hemby SE, McIntosh S, Leon F, Cutler SJ, McCurdy CR (2019) Abuse liability and therapeutic potential of the Mitragyna speciosa (Kratom) alkaloids mitragynine and 7-hydroxymitragynine. Addict Biol 24(5):874–885. https://doi.org/10.1111/adb.12639
- Henningfield JE, Fant RV, Wang DW (2018) The abuse potential of kratom according the 8 factors of the controlled substances act: implications for regulation and research. Psychopharmacology 235(2):573–589. https://doi.org/10.1007/s00213-017-4813-4
- Henningfield JE, Grundmann O, Babin JK, Fant RV, Wang DW, Cone EJ (2019) Risk of death associated with kratom use compared to opioids. Prev Med 128:105851. https://doi.org/10.1016/j.ypmed.2019.105851
- Henningfield JE, Grundmann O, Garcia-Romeu A, Swogger MT (2021) We need better estimates of kratom use prevalence. Am J Prev Med 62:132–133. https://doi.org/10.1016/j.amepre.2021.07.022
- Henningfield JE, Wang DW, Huestis MA (2022) Kratom abuse potential 2021: an updated eight factor analysis. Front Pharmacol 12:775073. https://doi.org/10.3389/fphar.2021.775073
- Hill R, Kruegel AC, Javitch JA, Lane JR, Canals M (2022) The respiratory depressant effects ofmitragynine are limited by its conversion to 7-OH mitragynine. Br J Pharmacol 179(14):3875–3885. https://doi.org/10.1111/bph.15832
- Huestis MA, Cone EJ, Pirnay SO, Umbricht A, Preston KL (2013) Intravenous buprenorphine and norbuprenorphine pharmacokinetics in humans. Drug Alcohol Depend 131(3):258–262. https://doi.org/10.1016/j.drugalcdep.2012.11.014
- Jagabalan Y, Zainal H, Al Ganaby A, Murugaiyah V, Ramanathan S (2019) Pharmacokinetic modeling of single dose Kratom (mitragynine) in rats. Conference Abstract: International Conference on Drug Discovery and Translational Medicine 2018, Putrajaya, Malaysia. Front Pharmacol. https://doi.org/10.3389/conf.fphar.2018.63.00087
- Kamble SH, Berthold EC, King TI, Raju Kanumuri SR, Popa R, Herting JR, León F, Sharma A, McMahon LR, Avery BA, McCurdy CR (2021) Pharmacokinetics of eleven kratom alkaloids following an oral dose of either traditional or commercial kratom products in rats. J Nat Prod 84(4):1104–1112. https://doi.org/10.1021/acs.jnatprod.0c01163
- Kruegel AC, Gassaway MM, Kapoor A, Váradi A, Majumdar S, Filizola M, Javitch JA, Sames D (2016) Synthetic and receptor signaling explorations of the Mitragyna alkaloids: mitragynine as an atypical molecular framework for opioid receptor modulators. J Am Chem Soc 138(21):6754–6764. https://doi.org/10.1021/jacs.6b00360
- Kruegel AC, Uprety R, Grinnell SG, Langreck C, Pekarskaya EA, Le Rouzic V, Ansonoff M, Gassaway MM, Pintar JE, Pasternak GW, Javitch JA, Majumdar S, Sames D (2019) 7-Hydroxymitragynine is an active metabolite of mitragynine and a key mediator of its analgesic effects. ACS Cent Sci 5(6):992–1001. https://doi.org/10.1021/acscentsci.9b00141
- Macko E, Weisbach JA, Douglas B (1972) Some observations on the pharmacology of mitragynine. Arch Int Pharmacodyn Ther 198(1):145
- Maxwell EA, King TI, Kamble SH, Raju KS, Berthold EC, León F, Avery BA, McMahon LR, McCurdy CR, Sharma A (2020) Pharmacokinetics and safety of mitragynine in beagle dogs. Planta Med 86(17):1278–1285. https://doi.org/10.1055/a-1212-5475
- McCurdy C, Grundmann O, McLaughlin J (2020) Kratom Resources. Department of Pharmacodynamics, College of Pharmacy, University of Florida. https://pd.pharmacy.ufl.edu/research/kratom/. Accessed 8 March 2022
- Nair AB, Jacob S (2016) A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 7(2):27–31. https://doi.org/10.4103/0976-0105.177703
- National Academy of Sciences (2011) Guide for the Care and Use of Laboratory Animals: Eighth Edition, 2011. Washington, DC: The National Academies Press. https://doi.org/10.17226/12910.
- National Institute on Drug Abuse (2020). Kratom Drug Facts. National Institutes of Health, Bethesda, MD. https://www.drugabuse.gov/publications/drugfacts/kratom. Accessed 8 March 2022
- Obeng S, Wilkerson JL, León F, Reeves ME, Restrepo LF, Gamez-Jimenez LR, Patel A, Pennington AE, Taylor VA, Ho NP, Braun T, Fortner JD, Crowley ML, Williamson MR, Pallares VLC, Mottinelli M, Lopera-Londoño C, McCurdy CR, McMahon LR, Hiranita T (2021) Pharmacological comparison of mitragynine and 7-hydroxymitragynine: in vitro affinity and efficacy for μ-opioid receptor and opioid-like behavioral effects in rats. J Pharmacol Exp Ther 376(3):410–427. https://doi.org/10.1124/jpet.120.000189
- O’Brien CP (2015). Drug addiction. In: Brunton LL, Chabner BA, Knollmann BC. eds. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 12e. McGraw Hill. https://accessmedicine.mhmedical.com/content.aspx?bookid=1613§ionid=102159667
- Olsen EO, O’Donnell J, Mattson CL, Schier JG, Wilson N (2019) Notes from the field: unintentional drug overdose deaths with kratom detected—27 states, July 2016–December 2017. MMWR Morb Mortal Wkly Rep 68(14):326–327 https://doi.org/10.15585/mmwr.mm6814a2
- Palamar JJ (2021) Past-Year kratom use in the U.S.: Estimates from a nationally representative sample. Am J Prev Med 61(2):240-245. https://doi.org/10.1016/j.amepre.2021.02.004
- Pergolizzi JV Jr, Raffa RB (2019) Safety and efficacy of the unique opioid buprenorphine for the treatment of chronic pain. J Pain Res 12:3299–3317. https://doi.org/10.2147/JPR.S231948
- Pergolizzi JV Jr, Scholten W, Smith KJ, Leighton-Scott J, Willis JC, Henningfield JE (2015) The unique role of transdermal buprenorphine in the global chronic pain epidemic. Acta Anaesthesiol Taiwan 53(2):71–76. https://doi.org/10.1016/j.aat.2015.06.001
- Prozialeck WC, Avery BA, Boyer EW, Grundmann O, Henningfield JE, Kruegel AC, McMahon LR, McCurdy CR, Swogger MT, Veltri CA, Singh D (2019) Kratom policy: the challenge of balancing therapeutic potential with public safety. Int J Drug Policy 70:70–77. https://doi.org/10.1016/j.drugpo.2019.05.003
- Prozialeck WC, Edwards JR, Lamar PC, Plotkin BJ, Sigar IM, Grundmann O, Veltri CA (2020) Evaluation of the mitragynine content, levels of toxic metals and the presence of microbes in kratom products purchased in the western suburbs of Chicago. Int J Environ Res Public Health 17(15):5512. https://doi.org/10.3390/ijerph17155512
- Prozialeck WC, Lamar PC, Krupp M 2nd, Moon M, Phelps LE, Grundmann O (2021) Kratom use within the context of the evolving opioid crisis and the COVID-19 pandemic in the United States. Front Pharmacol 12:729220. https://doi.org/10.3389/fphar.2021.729220
- Ramanathan S, McCurdy CR (2020) Kratom (Mitragyna speciosa): worldwide issues. Curr Opin Psychiatry 33(4):312–318. https://doi.org/10.1097/YCO.0000000000000621
- Reeve ME, Obeng S, Oyola FL, Behnke M, Restrepo LF, Patel A, Ho NP, Williamson MR, Jimenez LR, McCurdy CR, McMahon LR (2020) The adrenergic a2 receptor-mediated discriminative-stimulus effects of mitragynine, the primary alkaloid in Kratom (Mitragyna speciosa) in rats. FASEB J 34(S1):1. https://doi.org/10.1096/fasebj.2020.34.s1.05233
- Schimmel J, Amioka E, Rockhill K, Haynes CM, Black JC, Dart RC, Iwanicki JL (2021) Prevalence and description of kratom (Mitragyna speciosa) use in the United States: a cross-sectional study. Addiction 116(1):176–181. https://doi.org/10.1111/add.15082
- Schuman-Olivier Z, Hoeppner BB, Weiss RD, Borodovsky J, Shaffer HJ, Albanese MJ (2013) Benzodiazepine use during buprenorphine treatment for opioid dependence: clinical and safety outcomes. Drug Alcohol Depend 132(3):580–586. https://doi.org/10.1016/j.drugalcdep.2013.04.006
- Sharma A, Kamble SH, León F, Chear NJ, King TI, Berthold EC, Ramanathan S, McCurdy CR, Avery BA (2019) Simultaneous quantification of ten key Kratom alkaloids in Mitragyna speciosa leaf extracts and commercial products by ultra-performance liquid chromatography-tandemmass spectrometry. Drug Test Anal 11(8):1162–1171. https://doi.org/10.1002/dta.2604
- Sharma A, McCurdy CR (2021) Assessing the therapeutic potential and toxicity of Mitragyna speciosa in opioid use disorder. Expert Opin Drug Metab Toxicol 17(3):255–257. https://doi.org/10.1080/17425255.2021.1853706
- Singh D, Narayanan S, Vicknasingam B (2016) Traditional and non-traditional uses of Mitragynine (Kratom): a survey of the literature. Brain Res Bull 126(Pt 1):41–46. https://doi.org/10.1016/j.brainresbull.2016.05.004
- Suhaimi FW, Hassan Z, Mansor SM, Müller CP (2021) The effects of chronic mitragynine (Kratom) exposure on the EEG in rats. Neurosci Lett 745:135632. https://doi.org/10.1016/j.neulet.2021.135632
- Swogger MT, Walsh Z (2018) Kratom use and mental health: a systematic review. Drug Alcohol Depend 183:134–140. https://doi.org/10.1016/j.drugalcdep.2017.10.012
- Swogger MT, Smith KE, Garcia-Romeu A, Grundmann O, Veltri CA, Henningfield J, Busch LY (2022) Understanding kratom use: a guide for healthcare providers. Front Pharmacol 13:801855. https://doi.org/10.3389/fphar.2022.801855
- Todd DA, Kellogg JJ, Wallace ED, Khin M, Flores-Bocanegra L, Tanna RS, McIntosh S, Raja HA, Graf TN, Hemby SE, Paine MF (2020) Chemical composition and biological effects of kratom (Mitragyna speciosa): in vitro studies with implications for efficacy and drug interactions. Sci Rep 10(1):19158. https://doi.org/10.1038/s41598-020-76119-w
- U.S. Department of Health and Human Services (2021) Overdose Prevention Strategy. https://www.hhs.gov/overdose-prevention/. Accessed 8 March 2022
- U.S. Drug Enforcement Administration (DEA) (2016) DEA Rulemaking Docket, Comment on FR Doc # 2016–24659, Schedules of Controlled Substances: Temporary Placement of Mitragynine and 7-Hydroxymitragynine into Schedule I. Public comments on FR Doc # 2016–24659 (23,220 public comments). https://www.regulations.gov/docket/DEA-2016-0015/comments. Accessed 8 March 2022
- Umbricht A, Huestis MA, Cone EJ, Preston KL (2004) Effects of high-dose intravenous buprenorphine in experienced opioid abusers. J Clin Psychopharmacol 24(5):479–487. https://doi.org/10.1097/01.jcp.0000138766.15858.c6
- Underwood W, Anthony R (2020) AVMA guidelines for the euthanasia of animals: 2020 edition. American Veterinary Medical Association. https://www.spandidos-publications.com/var/AVMA_euthanasia_guidelines_2020.pdf. Accessed 9 March 2022
- United Nations Commission on Narcotic Drugs (2021) Implementation of the international drug control treaties: changes in the scope of control of substances Summary of assessments, findings and recommendations of the 44th World Health Organization’s (WHO) Expert Committee on Drug Dependence (ECDD), 11–15 October 2021: Kratom, mitragynine, 7-hydroxymitragynine. https://www.unodc.org/documents/commissions/CND/CND_Sessions/CND_64Reconvened/ECN72021_CRP12_V2108992.pdf. Accessed 10 March 2022
- Váradi A, Marrone GF, Palmer TC, Narayan A, Szabó MR, Le Rouzic V, Grinnell SG, Subrath JJ, Warner E, Kalra S, Hunkele A, Pagirsky J, Eans SO, Medina JM, Xu J, Pan YX, Borics A, Pasternak GW, McLaughlin JP, Majumdar S (2016) Mitragynine/corynantheidine pseudoindoxyls as opioid analgesics with mu agonism and delta antagonism, which do not recruit β-arrestin-2. J Med Chem 59(18):8381–8397. https://doi.org/10.1021/acs.jmedchem.6b00748
- Veltri C, Grundmann O (2019) Current perspectives on the impact of Kratom use. Subst Abuse Rehabil 10:23–31. https://doi.org/10.2147/SAR.S164261
- Vicknasingam B, Narayanan S, Beng GT, Mansor SM (2010) The informal use of ketum (Mitragyna speciosa) for opioid withdrawal in the northern states of peninsular Malaysia and implications for drug substitution therapy. Int J Drug Policy 21(4):283–288. https://doi.org/10.1016/j.drugpo.2009.12.003
- Wilson LL, Harris HM, Eans SO, Brice-Tutt AC, Cirino TJ, Stacy HM, Simons CA, León F, Sharma A, Boyer EW, Avery BA (2020) Lyophilized kratom tea as a therapeutic option for opioid dependence. Drug Alcohol Depend 216:108310. https://doi.org/10.1016/j.drugalcdep.2020.108310
- World Health Organization, Expert Committee on Drug Dependence (WHO ECDD) (2021) Pre-review report: kratom (Mitragyna speciosa), mitragynine, and 7-hydroxymitraginine for the forty-fourth meeting of the ECDD, Geneva, 11–15 October 2021. https://www.who.int/publications/m/item/kratom-mitragynine-7-hydroxymitragynine-critical-review-report. Accessed 8 March 2022
- Xu L, Chockalingam A, Stewart S, Shea K, Matta MK, Narayanasamy S, Pilli NR, Volpe DA, Weaver J, Zhu H, Davis MC, Rouse R (2020) Developing an animal model to detect drug-drug interactions impacting drug-induced respiratory depression. Toxicol Rep 7:188–197. https://doi.org/10.1016/j.toxrep.2020.01.008
- Xu L, Krishna A, Stewart S, Shea K, Racz R, Weaver JL, Volpe DA, Pilli NR, Narayanasamy S, Florian J, Patel V, Matta MK, Stone MB, Zhu H, Davis MC, Strauss DG, Rouse R (2021) Effects of sedative psychotropic drugs combined with oxycodone on respiratory depression in the rat. Clin Transl Sci 14(6):2208–2219. https://doi.org/10.1111/cts.13080
- Yue K, Kopajtic TA, Katz JL (2018) Abuse liability of mitragynine assessed with a self-administration procedure in rats. Psychopharmacology 235(10):2823–2829. https://doi.org/10.1007/s00213-018-4974-9
Acknowledgements
The authors express their appreciation to Daniel Wang and Yolanda Green for their editorial assistance with the manuscript and Gina Bittner for the development of the figures and tables.
Funding
This study was funded without restrictions by the American Kratom Foundation (AKF), a 501(c)3 nonprofit entity established by the American Kratom Association to promote science and policy research on plants and their potential health benefits. AKF had no input into the design of the study, evaluation of results, or publication of this article.
Notice:
The above article is a repost. Please refer to the source provided to go to the original article. Kratomlords.com, it’s holding company, and it’s owners do not endorse any published content provided herein.
Kratomlords.com strongly recommends following FDA guidelines regarding Kratom. Please refer to the following link for further information.
www.fda.gov/news-events/public-health-focus/fda-and-kratom